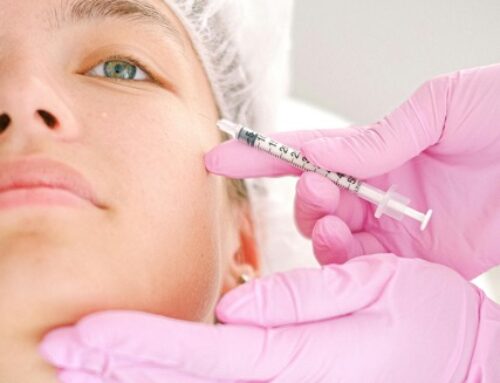
Obagi saypha MagIQ, the newly FDA-approved hyaluronic acid (HA) gel filler, is set to become a significant addition to the U.S. aesthetic market and will be available starting in 2026. The filler headlines Obagi Medical’s expansion into injectable products, offering a novel approach to dermal fillers for patients seeking natural-looking results and safety proven across all skin types.
FDA Approval and Science-backed Credentials
Obagi saypha MagIQ gained FDA clearance following a pivotal study focused on nasolabial fold (NLF) correction. The trial included representation from all Fitzpatrick Skin Types and met both primary and several secondary endpoints, underscoring reliable efficacy and safety among diverse patient populations.
Key Technology: MACRO Core Innovation
What sets saypha MagIQ apart is its MACRO Core Technology, forming a stable 3D HA matrix designed for smooth gel distribution and a predictable injection force and swelling profile. This technology enables practitioners to achieve consistent, precise, and natural outcomes, minimizing unpredictability during treatment and enhancing both safety and satisfaction.
Impact for Patients and Providers
The launch marks the first of several new products (including saypha ChIQ) planned for Obagi Medical’s U.S. portfolio, reflecting the brand’s commitment to evidence-based innovation and patient-centric care in aesthetic dermatology. This development positions Obagi saypha MagIQ as an exciting FDA-approved alternative for those considering filler treatments, particularly for nasolabial fold correction, with a strong emphasis on safety, effectiveness, and patient satisfaction.
References:
- The Dermatology Digest, U.S. FDA Nod for Obagi’s First Filler, Saypha MagIQ